https://www.youtube.com/@psyopchannel13 https://gab.com/boxoffrogs https://rumble.com/c/c-2352810 https://odysee.com/@HNiCC:1
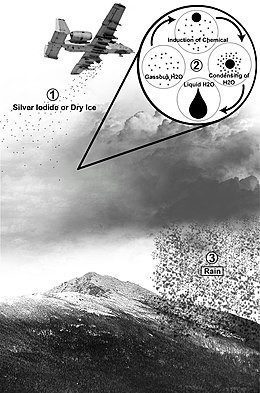
Except it's not silver iodine... it's aluminum, strontium, and beryllium... all much cheaper and much more toxic.
https://www.youtube.com/@psyopchannel13 https://gab.com/boxoffrogs https://rumble.com/c/c-2352810 https://odysee.com/@HNiCC:1
Silver nanoparticles produce a stronger plasmon resonance than gold nanoparticles it can be due to the large reflectivity from the metal surface. TEM images indicate that change in the size of nanoparticles with increasing the laser fluence is in good agreement with the Mie theory.
Magnetic hyperthermia aims to produce the local heating by a magnetically-mediated heating of low-frequency electromagnetic waves, through the power absorption by magnetic nanoparticles. This technique is one of the most important approaches to induce the local heating by low electromagnetic radiation.
In addition, an applied magnetic field can change the magnetic moment of the object itself; for example by magnetizing it. This phenomenon is known as magnetism. An applied magnetic field can flip the magnetic dipoles that make up the material causing both paramagnetism and ferromagnetism. Additionally, the magnetic field can affect the currents that create the magnetic fields (such as the atomic orbits) which causes diamagnetism.
The vicinity of metallic nanostructures that provides intense optical fields is termed as “hot spot”. Any molecule in close proximity to these hot spots will give rise to an increased surface-enhanced Raman spectroscopic (SERS) signal. We have synthesized Ag core−Au shell (core−shell) nanoparticles (NP) with nanopores, which act as hot spots in the SERS measurements. We have demonstrated a large enhancement in SERS studies of various molecules using core−shell NP with hot spots, which is better than using silver nanoparticles. The core−shell NP with hot spots can be used for ultratrace analysis of important biomolecules such as histone acetyltranferase p300, a human transcriptional coactivator protein. The core−shell NP does not change the biomolecule's physical and chemical property upon adsorption, which makes it biocompatible. The core−shell NP carrying hot spots with high SERS enhancement would be ideally suited for in vivo studies of biological systems.
Plasmonic optical trapping is based on the enhanced electromagnetic field by SPs and have two typical modes: one mode is using the focused laser to trap plasmonic nanoparticles while the other mode is using the plasmonic nanostructure to trap dielectric nanoparticles.
This thesis focuses on cavity optomechanics with an optically levitated dielectric ob- ject.
https://d-nb.info/1045023477/34
Optical resonators with whispering-gallery modes-part II: applications | IEEE Journals & Magazine | IEEE Xplore
We review photonic applications of dielectric whispering-gallery mode (WGM) resonators-tracing the growth of the technology from experiments with levitating droplets of aerosols to ultrahigh-Q solid state crystalline and integrated on-chip microresonators.
https://ieeexplore.ieee.org/document/1588879Optical nonlinearity involved switching draws an important consideration in nonlinear optical studies. Based on that, we explored nonlinear absorption processes in silver nanoparticles synthesized by liquid phase laser ablation technique employing a second harmonic wavelength (532 nm) of Q switched Nd:YAG laser pulses with 7 ns pulse width and 10 Hz repetition rates. The typical surface plasmon resonance induced absorption (~418 nm) confirmed the formation of Ag NPs. The Z-scan technique was used to study the nonlinear optical processes, employing the same laser system used for ablation. Our study reveals that there is an occurrence of a saturable to reverse saturable absorption switching activity in the Ag nanoparticles, which is strongly on-axis input intensity dependent as well. The closed aperture Z-scan analysis revealed the self-defocusing nature of the sample.
Supersaturation and Crystallization. The Driving Force For Crystallization. Supersaturation occurs when a solution contains more solute than should be possible thermodynamically, given the conditions of the system. Supersaturation is considered a major driver for crystallization.
There are two types of nucleation namely the homogeneous or spontaneous nucleation and heterogeneous nucleation. This phenomenon happens when nuclei are formed perfectly in a clean solution where there are no any foreign particles.
The amount of water that can exist as vapor in a given volume increases with the temperature. When the amount of water vapor is in equilibrium above a flat surface of water the level of vapor pressure is called saturation and the relative humidity is 100%. At this equilibrium there are equal numbers of molecules evaporating from the water as there are condensing back into the water. If the relative humidity becomes greater than 100%, it is called supersaturated. Supersaturation occurs in the absence of condensation nuclei.[citation needed]
Since the saturation vapor pressure is proportional to temperature, cold air has a lower saturation point than warm air. The difference between these values is the basis for the formation of clouds. When saturated air cools, it can no longer contain the same amount of water vapor. If the conditions are right, the excess water will condense out of the air until the lower saturation point is reached. Another possibility is that the water stays in vapor form, even though it is beyond the saturation point, resulting in supersaturation.[citation needed]
Supersaturation of more than 1–2% relative to water is rarely seen in the atmosphere, since cloud condensation nuclei are usually present.[26] Much higher degrees of supersaturation are possible in clean air, and are the basis of the cloud chamber.
A cloud chamber consists of a sealed environment containing a supersaturated vapour of water or alcohol. An energetic charged particle (for example, an alpha or beta particle) interacts with the gaseous mixture by knocking electrons off gas molecules via electrostatic forces during collisions, resulting in a trail of ionized gas particles. The resulting ions act as condensation centers around which a mist-like trail of small droplets form if the gas mixture is at the point of condensation. These droplets are visible as a "cloud" track that persists for several seconds while the droplets fall through the vapor. These tracks have characteristic shapes. For example, an alpha particle track is thick and straight, while a beta particle track is wispy and shows more evidence of deflections by collisions.
Cloud chambers played a prominent role in experimental particle physics from the 1920s to the 1950s, until the advent of the bubble chamber.. .
A bubble chamber is a vessel filled with a superheated transparent liquid (most often liquid hydrogen) used to detect electrically charged particles moving through it. It was invented in 1952 by Donald A. Glaser,[1] for which he was awarded the 1960 Nobel Prize in Physics.[2] Supposedly, Glaser was inspired by the bubbles in a glass of beer; however, in a 2006 talk, he refuted this story, although saying that while beer was not the inspiration for the bubble chamber, he did experiments using beer to fill early prototypes.[3]
An ultrasonic hydrogen bubble chamber has been constructed. The optimum temperature for the chamber operation is found by measuring the ultrasonic visible cavitation in liquid hydrogen. The observed values of the cavitation threshold under irradiation by gamma-rays from 60Co are the same order of magnitude as the values estimated on the basis of the thermal spike theory and the discrepancy between them can be reasonably explained. A high energy electron beam has been injected to the chamber and straight tracks have been successfully photographed.
A supersaturated solution is a solution that contains more than the maximum amount of solute that is capable of being dissolved at a given temperature. The recrystallization of the excess dissolved solute in a supersaturated solution can be initiated by the addition of a tiny crystal of solute, called a seed crystal.
Controlling particle size is essential for crystal quality in the chemical and pharmaceutical industry. Several articles illustrate the potential of ultrasound to tune this particle size during the crystallization process. This paper investigates how ultrasound can control the particle size distribution (PSD) of acetaminophen crystals by continuous seed generation in a tubular crystallizer followed by batch growth. It is demonstrated that the supersaturation ratio at which ultrasound starts seed generation has a substantial effect on the final PSD while the applied power is insignificant in the studied conditions.
The higher the supersaturation ratio, the smaller the final crystals become up to a supersaturation ratio of 1.56. Furthermore, it was shown that ultrasound can also impact the final PSD when applied during the growth phase. Frequencies of 850 kHz or below reduce the final particle size; the lower the applied frequency, the smaller the crystals become. In conclusion, one could state that ultrasound is able to control the particle size during seed generation and subsequent growth until the final particle size.