Got a calling from God the Father... "SERVE GOD AND THOSE HE LOVES." I hope to always heed that. Happily married to a Marine Veteran.
(...)"Whom ever is navigating these storms and waters with us, we are aboard, and on deck, because alas~ WWG1WGA" ~R
EGFP Sequence (717bp without STOP CODON):
5'-
ATGGTGAGCAAGGGCGAGGAGCTGTTCACCGGGGTGGTGCCCATCCTGGTCGAGCTGGACGGCGACGTAAACGGCCACAA GTTCAGCGTGTCCGGCGAGGGCGAGGGCGATGCCACCTACGGCAAGCTGACCCTGAAGTTCATCTGCACCACCGGCAAGC TGCCCGTGCCCTGGCCCACCCTCGTGACCACCCTGACCTACGGCGTGCAGTGCTTCAGCCGCTACCCCGACCACATGAAG CAGCACGACTTCTTCAAGTCCGCCATGCCCGAAGGCTACGTCCAGGAGCGCACCATCTTCTTCAAGGACGACGGCAACTA CAAGACCCGCGCCGAGGTGAAGTTCGAGGGCGACACCCTGGTGAACCGCATCGAGCTGAAGGGCATCGACTTCAAGGAGG ACGGCAACATCCTGGGGCACAAGCTGGAGTACAACTACAACAGCCACAACGTCTATATCATGGCCGACAAGCAGAAGAAC GGCATCAAGGTGAACTTCAAGATCCGCCACAACATCGAGGACGGCAGCGTGCAGCTCGCCGACCACTACCAGCAGAACAC CCCCATCGGCGACGGCCCCGTGCTGCTGCCCGACAACCACTACCTGAGCACCCAGTCCGCCCTGAGCAAAGACCCCAACG AGAAGCGCGATCACATGGTCCTGCTGGAGTTCGTGACCGCCGCCGGGATCACTCTCGGCATGGACGAGCTGTACAAG +STOP CODON-3'
(...)"Whom ever is navigating these storms and waters with us, we are aboard, and on deck, because alas~ WWG1WGA" ~R
What is the GFP nucleotide sequence?
Green fluorescent protein.
👍🏻
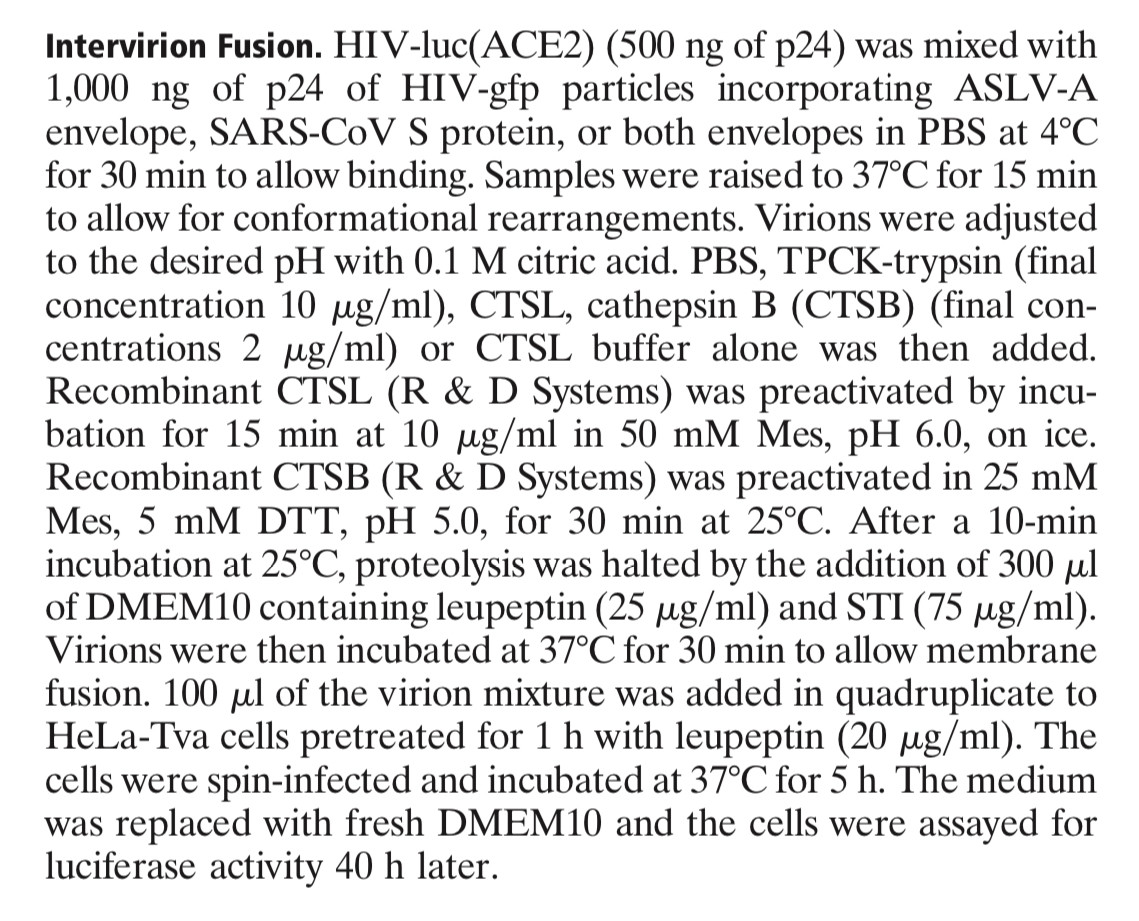
Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry
Graham Simmons*†, Dhaval N. Gosalia‡, Andrew J. Rennekamp*, Jacqueline D. Reeves*, Scott L. Diamond‡§, and Paul Bates*†
*Department of Microbiology, School of Medicine and Departments of ‡Bioengineering and §Chemical and Biomolecular Engineering, Institute for Medicine and Engineering, University of Pennsylvania, Philadelphia, PA 19104
Communicated by Harold E. Varmus, Memorial Sloan–Kettering Cancer Center, New York, NY, July 1, 2005 (received for review May 5, 2005)
Trypsin (EC 3.4.21.4) is a serine protease from the PA clan superfamily, found in the digestive system of many vertebrates, where it hydrolyzes proteins.[2][3] Trypsin is formed in the small intestine when its proenzyme form, the trypsinogen produced by the pancreas, is activated. Trypsin cuts peptide chains mainly at the carboxyl side of the amino acids lysine or arginine. It is used for numerous biotechnological processes. The process is commonly referred to as trypsin proteolysis or trypsinization, and proteins that have been digested/treated with trypsin are said to have been trypsinized.[4] Trypsin was discovered in 1876 by Wilhelm Kühne and was named from the Ancient Greek word for rubbing since it was first isolated by rubbing the pancreas with glycerin
Cathepsin L
Cathepsin L released from the lysosome into the newly formed autophagolysosomes degrades any trypsinogen which may be present and prevents its activation to trypsin, hence short-circuiting any inappropriate activation of other zymogen pro-enzymes.
From: Haschek and Rousseaux's Handbook of Toxicologic Pathology (Third Edition), 2013