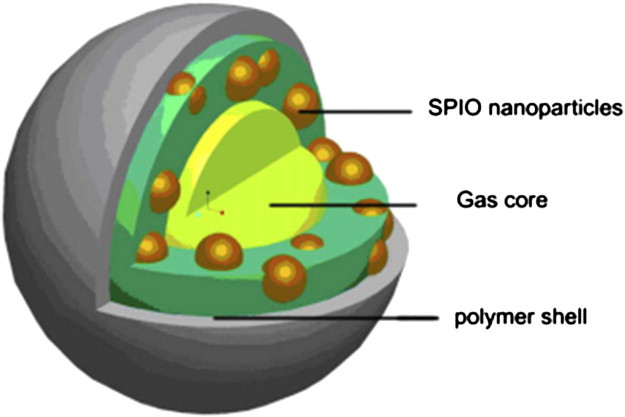
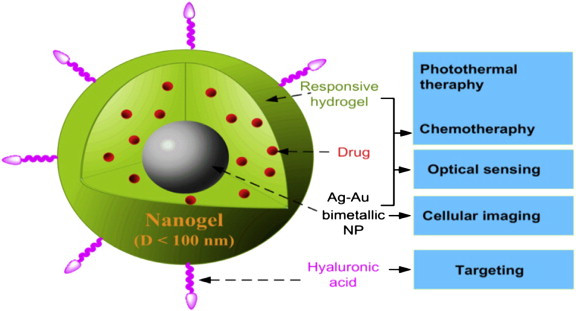
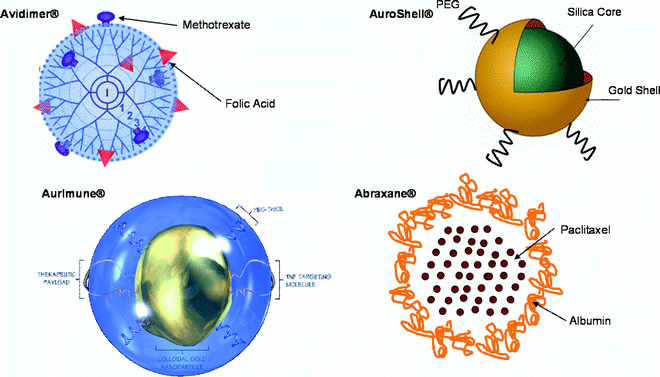
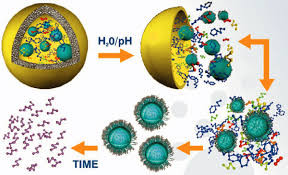
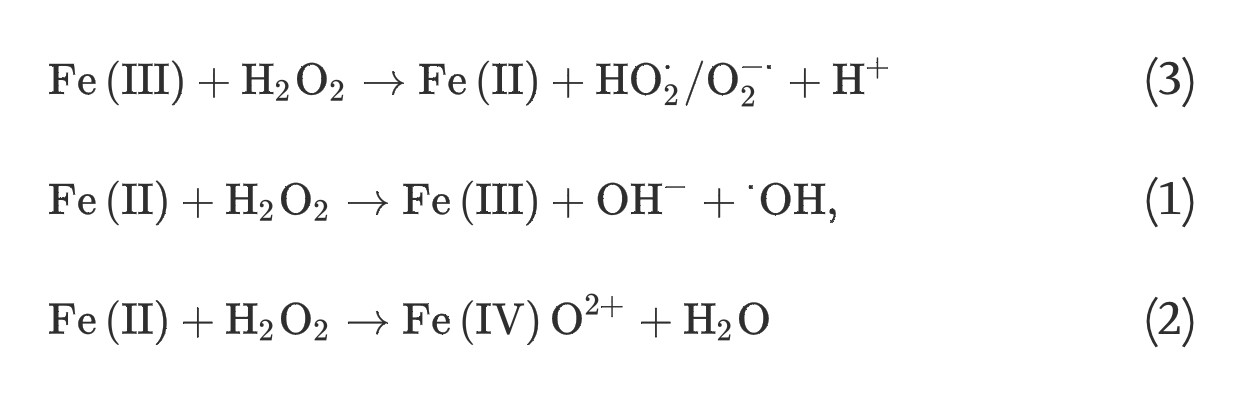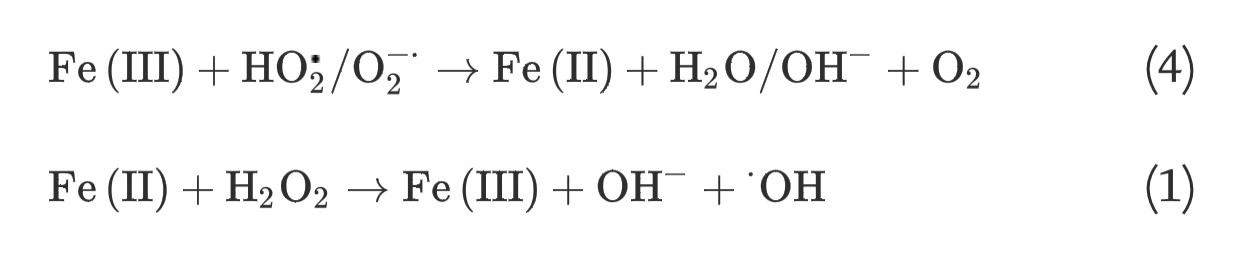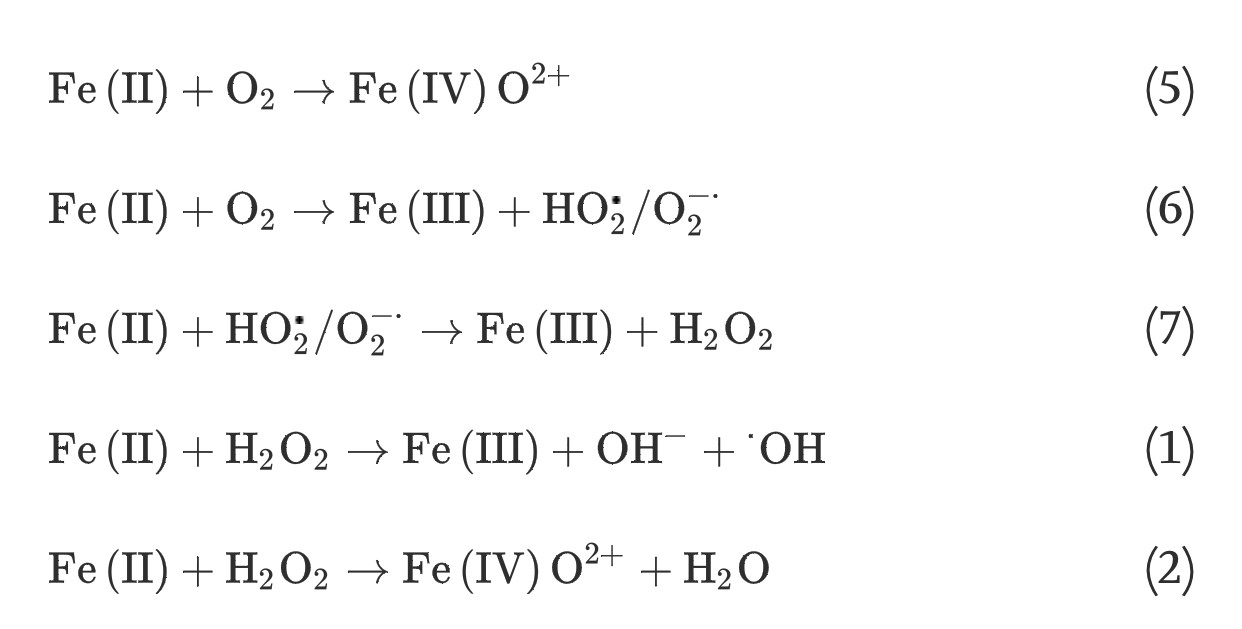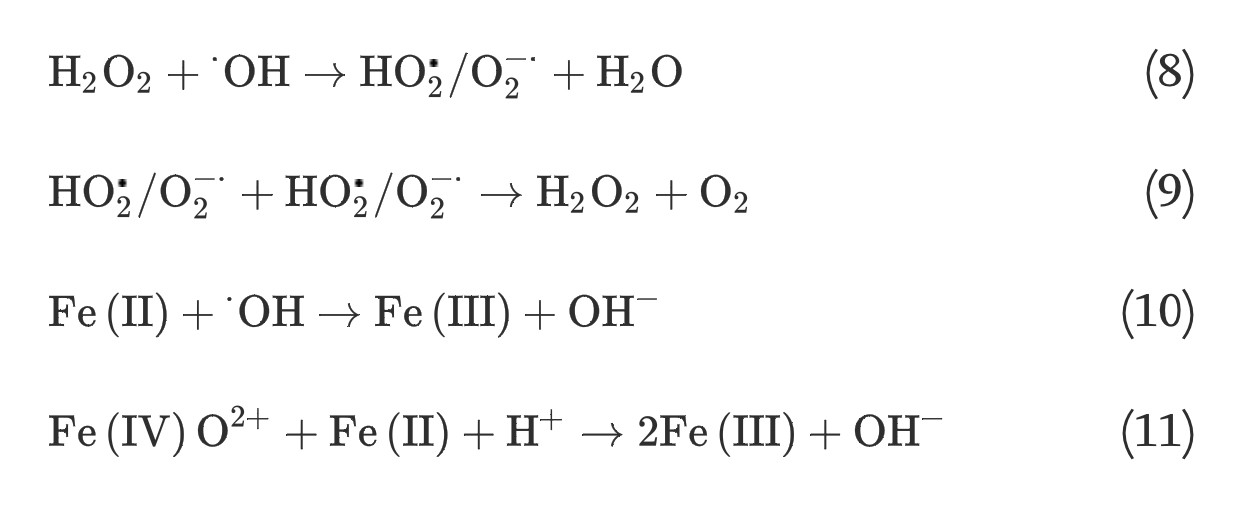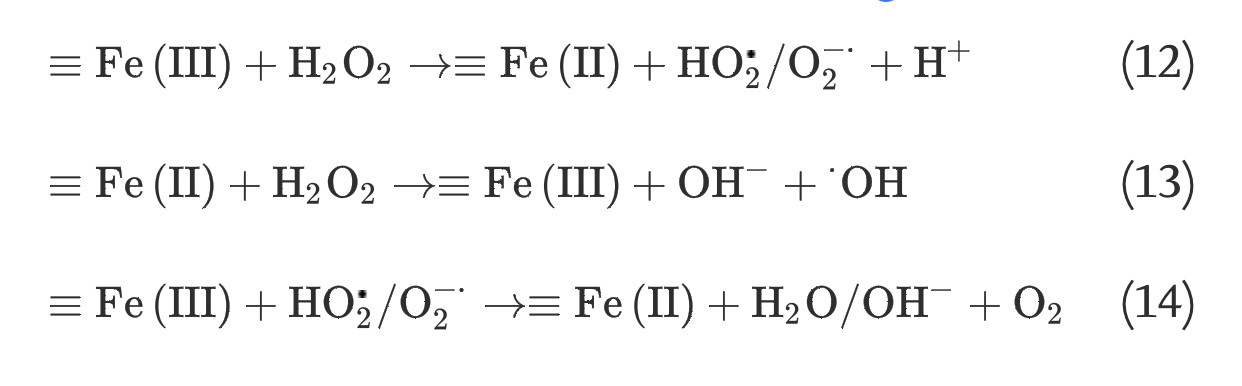I am a Information Treasure Hunter. I was sent to earth to teach Love and Kindness
A Mass Murderer.
Lipid peroxidation markers are elevated in autism, indicating that oxidative stress is increased in this disease. Levels of major antioxidant serum proteins, namely transferrin (iron-binding protein) and ceruloplasmin (copper-binding protein), are decreased in children with autism. There is a positive correlation between reduced levels of these proteins and loss of previously acquired language skills in children with autism. The alterations in ceruloplasmin and transferrin levels may lead to abnormal iron and copper metabolism in autism. The membrane phospholipids, the prime target of ROS, are also altered in autism. The levels of phosphatidylethanolamine (PE) are decreased, and phosphatidylserine (PS) levels are increased in the erythrocyte membrane of children with autism as compared to their unaffected siblings. Several studies have suggested alterations in the activities of antioxidant enzymes such as superoxide dismutase, glutathione peroxidase, and catalase in autism.
Additionally, altered glutathione levels and homocysteine/methionine metabolism, increased inflammation, excitotoxicity, as well as mitochondrial and immune dysfunction have been suggested in autism. Furthermore, environmental and genetic factors may increase vulnerability to oxidative stress in autism. Taken together, these studies suggest increased oxidative stress in autism that may contribute to the development of this disease. A mechanism linking oxidative stress with membrane lipid abnormalities, inflammation, aberrant immune response, impaired energy metabolism and excitotoxicity, leading to clinical symptoms and pathogenesis of autism is proposed.
2. ROS-related redox mechanisms of nano-iron metal and nano-iron oxides
Successive one-electron or two-electron reduction of molecular oxygen to water in the aqueous solution yields a series of ROS such as superoxide radicals (O2−/HO2radical dot), hydrogen peroxide (H2O2), and hydroxyl radicals (OHradical dot). Nano-iron metal and nano-iron oxides can be involved in these redox reactions as the reactant or catalyst via a homogeneous or heterogeneous means, based on dissolved iron species or solid surfaces, respectively. The standard reduction potentials (E0) of redox pairs covered in this review are cited from previous studies [13], [14], [15].
2.1. Homogeneous reactions
(1) The classic homogeneous Fenton reaction, which involves one-electron reduction of hydrogen peroxide by soluble ferrous iron species, generates hydroxyl radicals (E0 = +2.3 V) that are powerful enough to oxidize most organic molecules:
(1)
By convention, Fe(II) and Fe(III) represent all ferrous and ferric iron species, respectively.
(2) The non-radical mechanism for the homogeneous Fenton reaction involves the generation of ferryl-oxo complexes (E0 = +0.9 V), less powerful oxidants compared to hydroxyl radicals, via two-electron reduction of hydrogen peroxide with soluble ferrous iron species:
(2)
Based on the distinct oxidizing power of hydroxyl radicals and ferryl-oxo complexes, many studies have found that radical and non-radical Fenton reactions are competing reactions with a pH-dependent partitioning [16], [17], [18].
(3) The homogeneous Fenton-like reactions, which involve the generation of superoxide radicals, hydroxyl radicals, or ferryl-oxo complexes from hydrogen peroxide and soluble ferric iron species, consist of two steps; a slow one-electron reduction of ferric iron by hydrogen peroxide and a rapid generation of powerful oxidants via reaction (1), (2):
(3)
(1)
(2)
(3) The Haber-Weiss reaction, which involves generation of hydroxyl radicals from hydrogen peroxide and superoxide, can be catalyzed by soluble ferric iron species through two steps:
(4)
(1)
(4) The homogeneous Fe(II) autoxidation in the presence of molecular oxygen in the aqueous solution via single-step two-electron transfer or stepwise one-electron transfer reactions can generate oxidants of ferryl-oxo complexes or a series of ROS [19], [20]:
It has long been recognized that iron oxides can initiate heterogeneous redox reactions at the aqueous-solid interface. Zalma et al [22] reported that iron oxides including akaganeite, magnetite, crocidolite, and hematite were active in producing hydroxyl radicals in the presence of hydrogen peroxide by redox reactions initiated at the solid surface. Lin and Gurol [23] found that the kinetic model of catalytic decomposition of hydrogen peroxide by the granular goethite in aqueous solution at pH 7 was similar to the classical Langmuir-Hinshelwood rate model, which usually describes heterogeneous surface reactions, and proposed a redox mechanism of iron oxides based on the surface-complexation chemistry of surface-bound ferric [≡Fe(III)] and ferrous [≡Fe(II)] iron:
By comparing the surface-area-normalized redox activities of iron nanostructures with different sizes, many studies have found that size-dependent physicochemical surface properties also play an important role in the redox reactions of iron nanostructures. Wang and Zhang [36] found that surface-area-normalized reduction rate constants of zero-valent iron nanoparticles with specific surface area of 33.5 m2/g were calculated to be 10−100 times higher than those of microparticles with specific surface area of 0.9 m2/g. As reported by Anschutz and Penn [37], surface-area-normalized reduction dissolution rates for nanoparticles of 4 nm ferrihydrite and 5 nm × 64 nm goethite by hydroquinone are up to 20 times and two times faster than the rates for nanoparticles of 6 nm ferrihydrite and 22 nm × 367 nm goethite, respectively [37].
Cwiertny et al [38] demonstrated that the surface-area-normalized rate of oxalate-promoted dissolution of goethite was four times greater in nanorod suspensions than in microrod suspensions [38]. Similarly, after surface area normalization, the initial and steady state reductive dissolution rates for hematite nanoparticles with average sizes of 6.8 nm by ascorbic acid were about two times and one point five times of those with average size of 30.5 nm according to Echigo et al [39]. The surface physicochemical properties of smaller iron oxide particles thus seem to favor iron dissolution, so that more rapid induction of homogeneous redox reactions could be expected for iron oxide with smaller dimensions.
Additionally, specific size-dependent physicochemical surface properties of iron oxides have also been reported. According to Cornell and Schwertmann [40], particle size has a marked effect on the solubilities of iron oxides, due to the relatively high surface free energy of the compounds, and the solubility products (Ksp) of hematite and goethite could increase by two orders of magnitude when particle size decreases from 1 μm to 10 nm, as illustrated first by Langmuir [41] in 1971. Chernyshova et al [42] observed a regular increase in tetrahedral defects in the near surface regions of hematite with decreasing particle size, whereas surface defects were well recognized to affect reactivity of metal oxides. Gilbert et al [43] found that surface oxygen sites of 6 nm goethite nanoparticles are less coordinated by iron relative to bulk goethite, and proposed that this could consequently result in the increased redox reactivity of goethite nanoparticles.
During their biotransformation in living organisms, nanomaterials might encounter a wide range of bio-microenvironments, including both extracellular and intracellular biological fluids. Nanomaterials can be adsorbed on the skin, inhaled into the lung, ingested into the gastrointestinal tract, or injected into the blood, and then be internalized into cells of specific tissues through various endocytosis pathways. Within cells, many nanoparticles could end up in the lysosomes, but some of them that are capable of escaping from endosomes or lysosomes might appear in cytoplasm and other cellular organelles such as the Golgi body, mitochondria, and nucleus [54].
The ROS levels in animal blood have been recognized to rise under certain physiological conditions, such as breeding, hypertension, and inflammation [55]. Within cells, ROS are continuously produced mainly as by-products of electron transportation in the mitochondria, and may also appear in other cellular locations after escaping the effective antioxidant defense [2]. Therefore, in order to understand the biological effects of iron nanostructures, it is crucial to characterize ROS-related activities of nano-iron metal and nano-iron oxides under these bio-microenvironmental conditions.
4.2. Biogenic reducing agents
Body fluid maintains a reducing environment compared to iron oxides, and various biogenic reducing agents (e.g., glutathione, cysteine, ascorbic acid, NADH, NADPH, succinate, and ubiquinol) with a wide range of E0 are distributed in extracellular and intracellular biological fluids. Human plasma maintains a mean E0 of as low as −140 mV, but is still 90 mV more oxidized than the cytoplasmic pool. Among the cellular organelles, the mitochondrion is the most reducing cellular compartment with an E0 of about −318 mV, followed by lysosome (−240 mV), and endoplasmic reticulum (−189 mV) [65]. It is thus of great biological importance to consider the redox activities of iron nanostructures under reducing bio-microenvironmental conditions
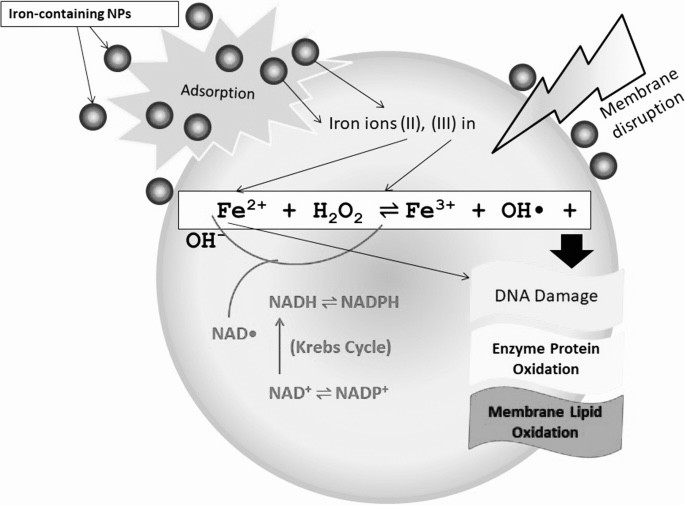
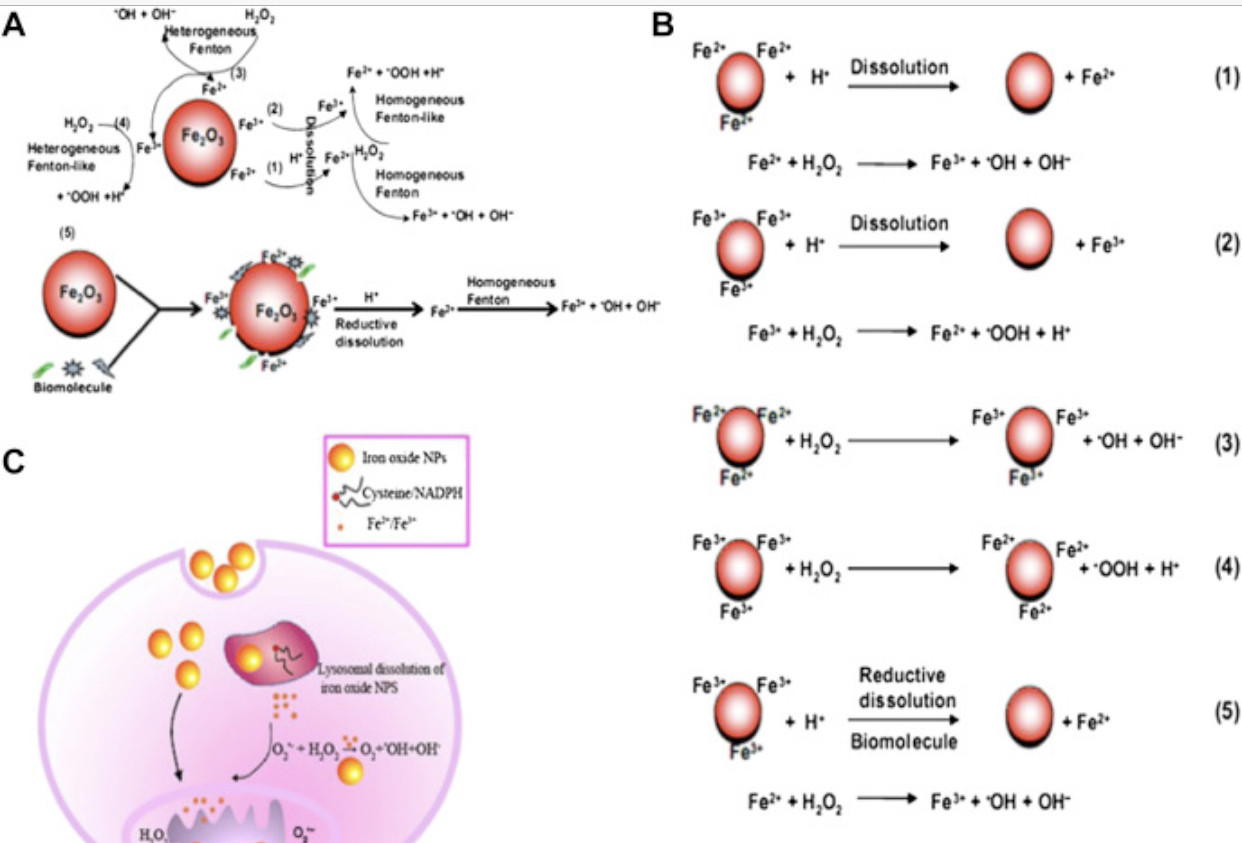
Reductive dissolution of iron oxide nanoparticles will inevitably induce more homogeneous Fenton reactions, which are much more efficient in producing damaging ROS than heterogeneous Fenton reactions (Fig. 2). Wang et al [51] revealed that significantly larger dissolved iron fractions were detected in solutions containing maghemite and hematite nanoparticles in the presence of cysteine and NADPH under all pH regimes, and they also observed that these fractions induced much more hydroxyl radicals from hydrogen peroxide than the solid surfaces. Substantial evidence also indicates that superparamagnetic iron oxide nanoparticles are gradually degraded in endosomes and lysosomes, where the dissolved iron species cause oxidative stress to induce severe membrane permeation and subsequent cellular dysfunction [66], [67], [68].
Fig. 2. Scheme for radical dotOH free radical generation by iron oxide nanoparticles (NPs) in bio-microenvironments. (A) radical dotOH generation at the nanobio interface of Fe2O3 NPs; (B) chemical processes of radical dotOH generation by Fe2O3 NPs; (C) intracellular radical dotOH free radical generation: dissolution or in situ reductive dissolution of magnetic iron oxide NPs may occur in the acidic lysosomal microenvironment, which depends on surface hydroxylation and surface Fe oxidation state of iron oxide NPs; the free Fe ions or NPs can react with hydrogen peroxide and superoxide in mitochondria and cytoplasm to produce highly reactive radical dotOH via homogeneous or heterogeneous Fe(II)/Fe(III) catalytic Fenton/Haber-Weiss type reaction.
In addition to those which function as reducing agents, a wide range of organic substances with diverse biological functionalities and chemical groups (such as carboxylate, phenolate, hydroxy, thiol, and amines) are also ubiquitous in biological fluids. The interactions of these organic substances with homogeneous or heterogeneous iron species can also influence the redox activities of iron nanostructures. According to Lipczynska-Kochany et al [64], anions including the above-mentioned phosphate and carbonate might influence hydrogen peroxide decomposition and oxidant generation through modifying the Fe(II) oxygenation rate and competitive scavenging of reactive intermediates.
As demonstrated by Valentine and Wang [61], Fukushima and Tatsumi [69], and Vione et al [70], humic acid could increase oxidant generation efficiencies of homogeneous or heterogeneous Fenton and Fenton-like systems through their carboxyl and phenolate groups. Kachur et al [19] found that hydroxyl radical formation during Fe(II) autoxidation was enhanced significantly by the addition of polyphosphates (tri- and tetrapolyphosphate and their adenosine derivatives), citrate, and acetic derivatives of EDTA, diethylenetriaminepentaacetic acid, triethylenetetraminehexaacetic acid, ethylenediamine-(N,N′)-diacetic acid, and nitrilotriacetic acid (NTA) with the generation rate of hydroxyl radicals showing an inverse correlation with the charge of the ferrous ion complex. Iwahashi et al [71] revealed that a series of organic acids, including quinolinic acid, α-picolinic acid, fusaric acid, and 2,6-pyridinedicarboxylic acid, could enhance the hydroxyl radical yield of the homogeneous Fenton system