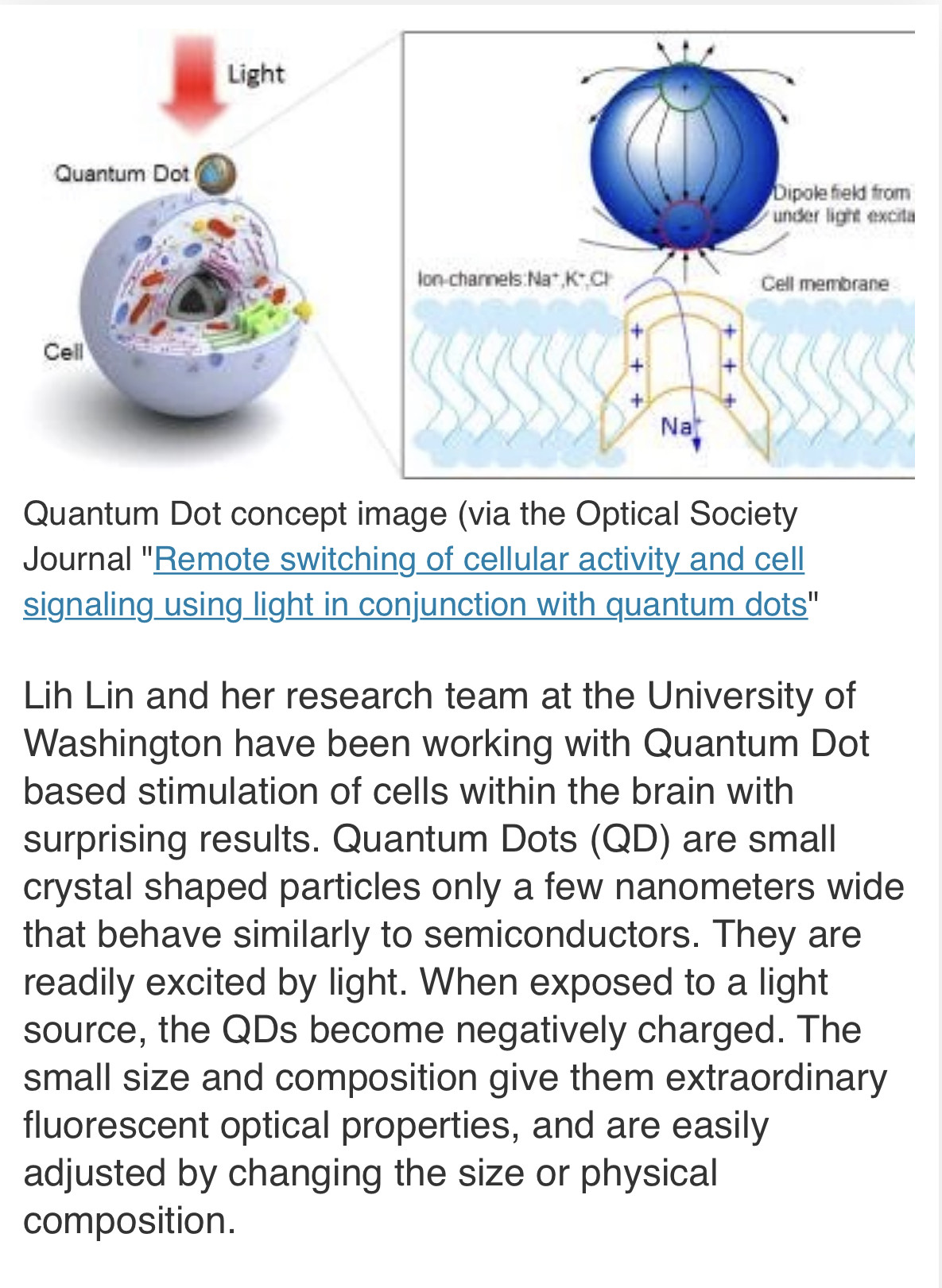
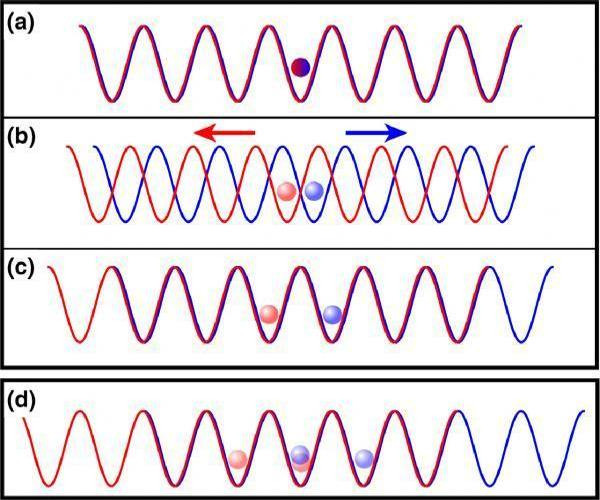
In response The Mac to his Publication
In response The Mac to his Publication
NOT A COINCIDENCE?
In response The Mac to his Publication
In response The Mac to his Publication
In response The Mac to his Publication
In response The Mac to his Publication
In response The Mac to his Publication
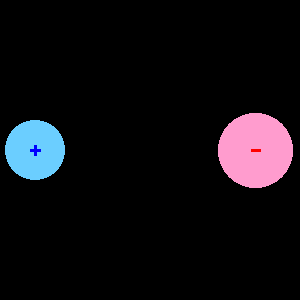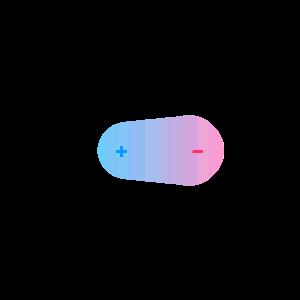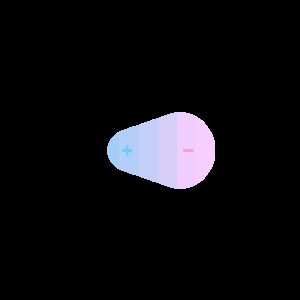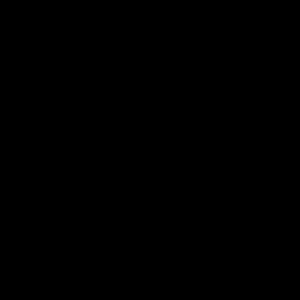
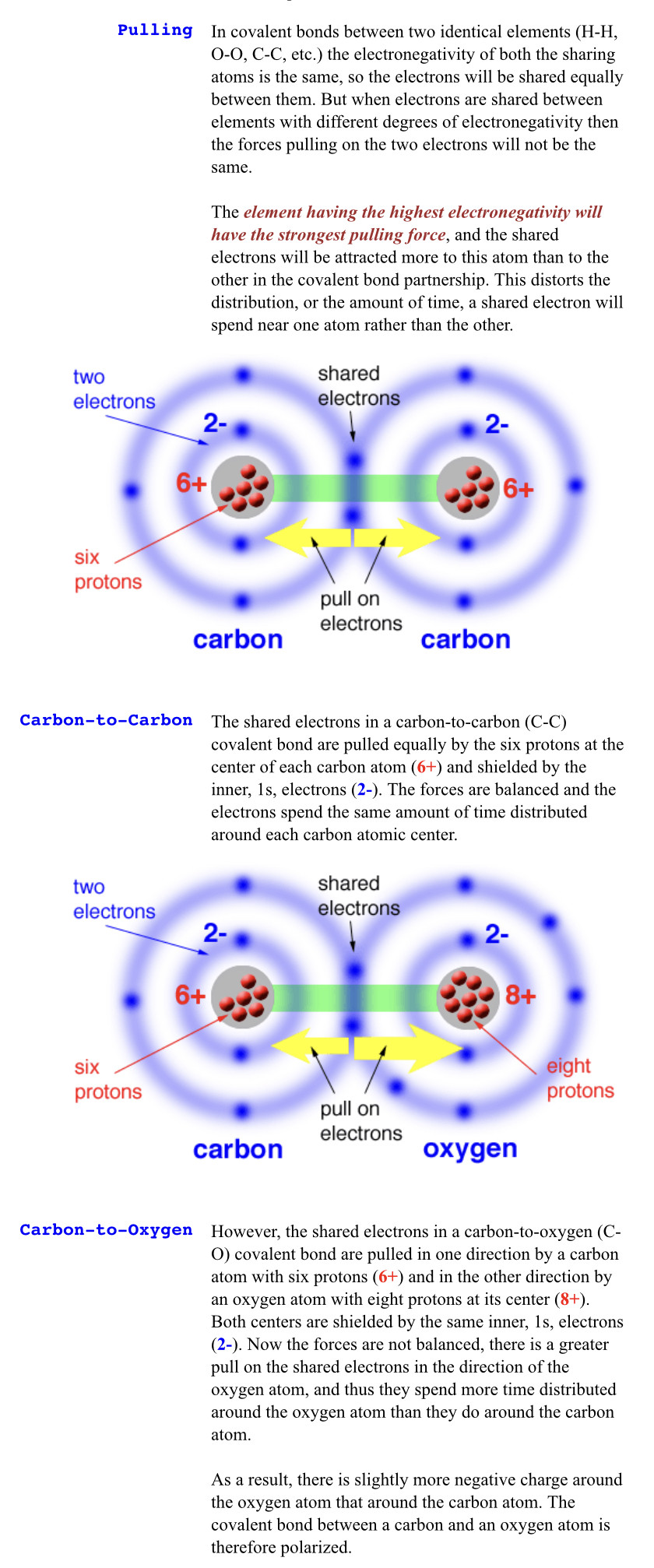
When two different atoms are bonded covalently, the shared electrons are attracted to the more electronegative atom of the bond, resulting in a shift of electron density toward the more electronegative atom. Such a covalent bond is polar, and will have a dipole (one end is positive and the other end negative).
In response Lisa Daigle to her Publication
In response The Mac to his Publication