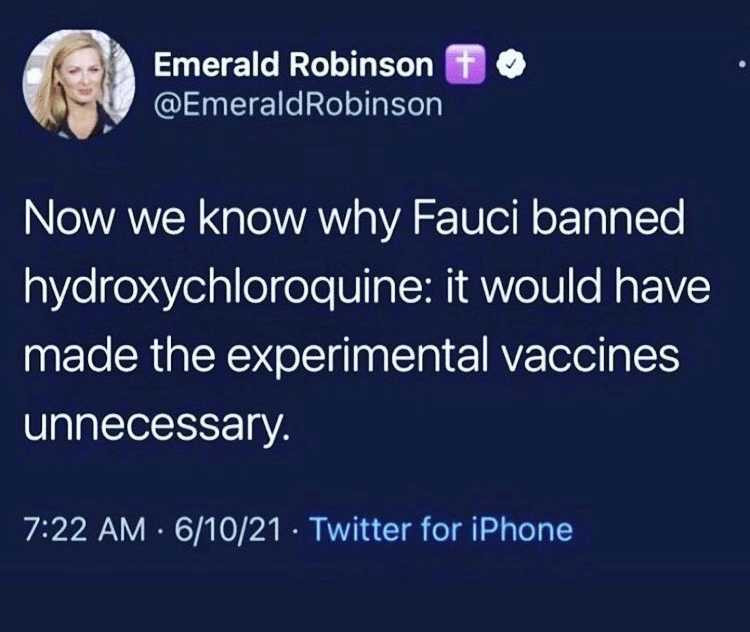
Fact. Research the definition of....EUA.
Heck, here ya go.....Emergency Use Authorization:
Under section 564 of the Federal Food, Drug, and Cosmetic Act (FD&C Act), when the Secretary of HHS declares that an emergency use authorization is appropriate, FDA may authorize unapproved medical products or unapproved uses of approved medical products to be used in an emergency to diagnose, treat, or prevent serious or life-threatening diseases or conditions caused by CBRN threat agents when certain criteria are met, including there are no adequate, approved, and available alternatives.
INCLUDING?!