Trump, Q, Deplorable, WWG1WGA, God wins, Save the CHILDREN!
Your info is fascinating. Could you make one post and explain it further?
🙂
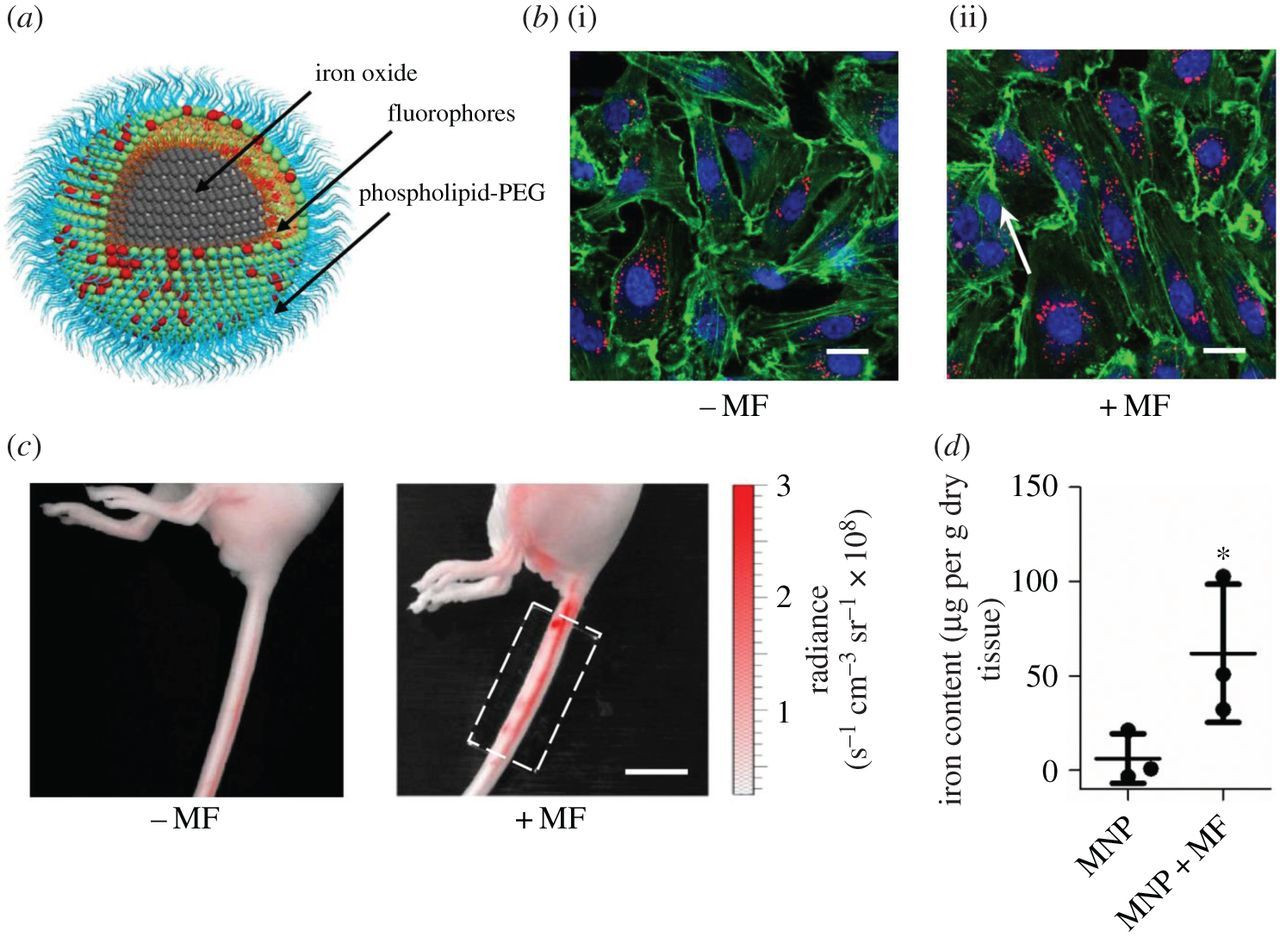
Magnetogenetics refers to a biological technique that involves the use of magnetic fields to remotely control cell activity.
In most cases, magnetic stimulation is transformed into either force (magneto-mechanical genetics) or heat (magneto-thermal genetics), which depends on the applied magnetic field.
Therefore, cells are usually genetically modified to express ion channels that are either mechanically or thermally gated.
As such, magnetogenetics is a cellular modulation method that uses a combination of techniques from magnetism and genetics to control activities of individual cells in living tissue – even within freely moving animals.
This technique is comparable to optogenetics, which is the manipulation of cell behavior using light. In magnetogenetics, magnetic stimulation is used instead of light, a characteristic that allows for a less invasive, less toxic, and wireless modulation of cell activity.
Iron oxide (Fe₃O₄) nanoparticles (IONPs) have received much attention for their utility in biomedical applications such as magnetic resonance imaging, drug delivery and hyperthermia.
Recent studies reported that IONPs induced cytotoxicity in mammalian cells.
However, little is known about the genotoxicity of IONPs following exposure to human cells. In this study, we investigated the cytotoxicity, oxidative stress and genotoxicity of IONPs in two human cell lines; skin epithelial A431 and lung epithelial A549. Prepared IONPs were polygonal in shape with a smooth surface and had an average diameter of 25 nm.
IONPs (25-100 μg/ml) induced dose-dependent cytotoxicity in both types of cells, which was demonstrated by cell viability (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide) and lactate dehydrogenase leakage assays. IONPs were also found to induce oxidative stress in a dose-dependent manner, evident by depletion of glutathione and induction of reactive oxygen species (ROS) and lipid peroxidation.
Comet assay revealed that level of DNA damage was higher with concentration of IONPs in both types of cells. Quantitative real-time PCR analysis showed that following the exposure of cells to IONPs, the expression levels of mRNA of caspase-3 and caspase-9 genes were higher.