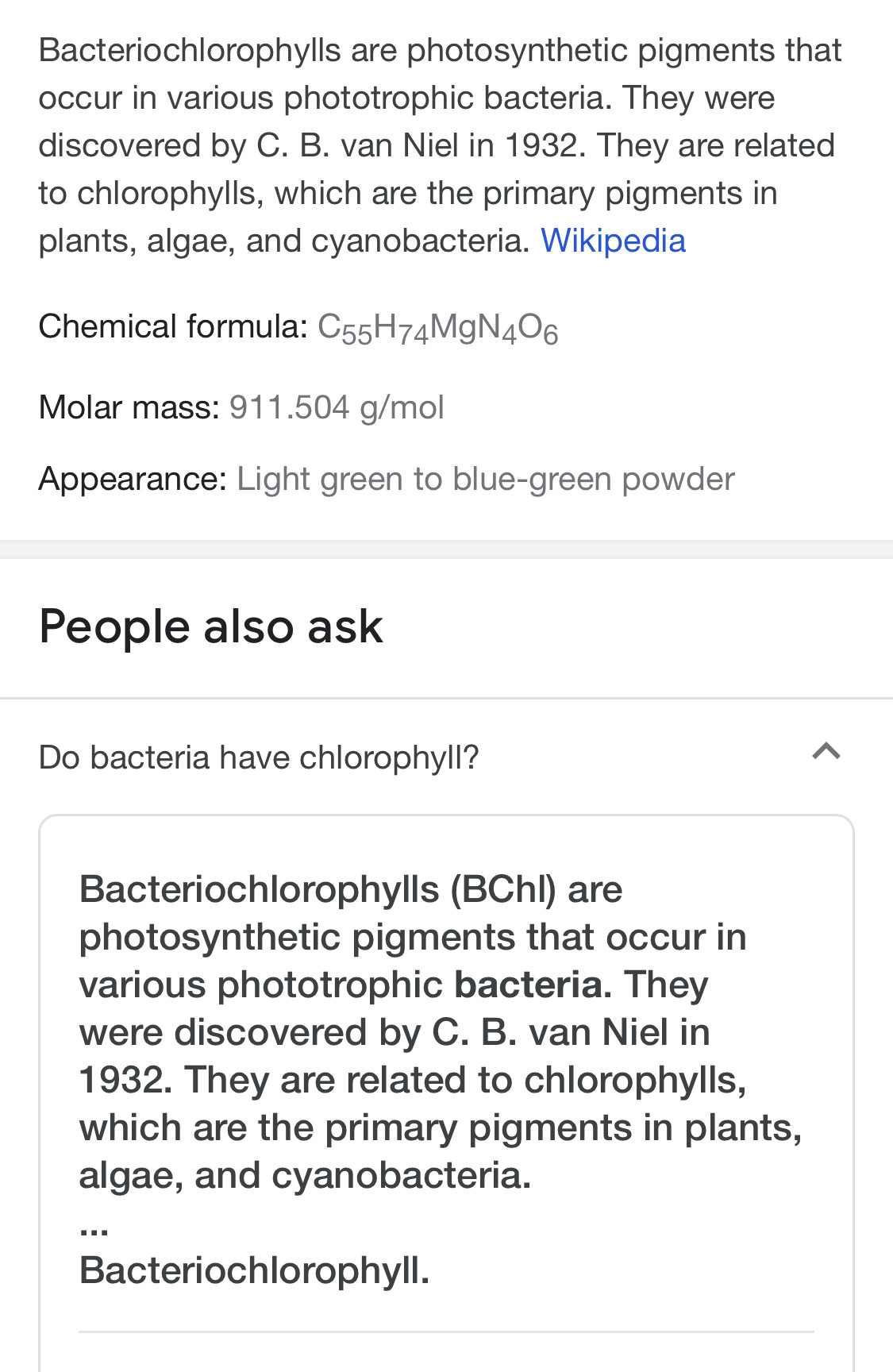
👋🏻
An automated procedure was developed to determine the geometrical and chemical interactions of crystalline virus particles using the crystal parameters, particle position, orientation, and atomic coordinates for an icosahedral asymmetric unit.
Two applications of the program are reported: (1) An analysis of a novelpseudo-rhombohedral (R32) symmetry present in the monoclinic crystal lattices of both Nodamura Virus (NOV) and Coxsackie virus B3 (CVB3). The study shows that in both cases the interactions between particles is substantially increased by minor deviations from exact R32 symmetry and that only particles with the proper ratio of dimensions along twofold and fivefold symmetry axes (such as southern bean mosaic virus) can achieve comparable buried surface area in the true R32 space group.
(2) An attempt was made to correlate biological function with remarkably conserved interparticle contact regions found in different crystal forms of three members of the nodavirus family, NOV, Flock House Virus (FHV), and Black Beetle Virus (BBV). Mutational evidence implicates the quasi-threefold region on the viral surface in receptor binding in nodaviruses and this region is dominant in particle contacts in all three virus crystals.
Examination of particle contacts in numerous crystal structures of viruses in the picornavirus superfamily showed that portions of the capsid surface known to interact with a receptor or serve as an epitope for monoclonal antibodies frequently stabilize crystal contacts.
A photoreceptor cell is a specialized type of neuroepithelial cell found in the retina that is capable of visual phototransduction. The great biological importance of photoreceptors is that they convert light (visible electromagnetic radiation) into signals that can stimulate biological processes.
chloroplast; plural noun: chloroplasts
a plastid in green plant cells which contains chlorophyll and in which photosynthesis takes place.
Viruses can infect bacteria.
What is the Difference between Viruses and Bacteria?
Living or Not
Viruses are not living organisms, bacteria are.
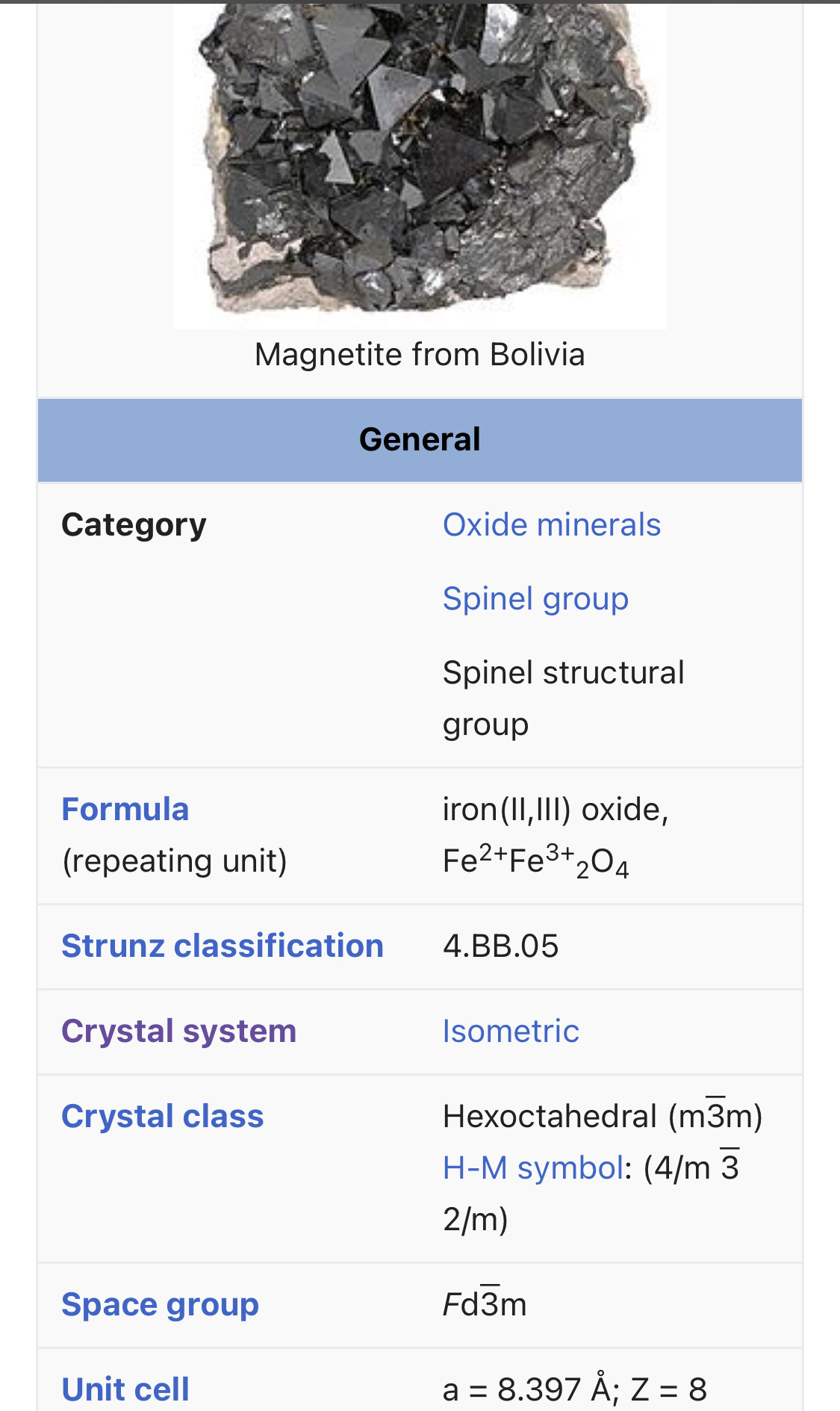
Magnetite is a mineral and one of the main iron ores, with the chemical formula Fe3O4. It is one of the oxides of iron, and is ferrimagnetic; it is attracted to a magnet and can be magnetized to become a permanent magnet itself. It is the most magnetic of all the naturally occurring minerals on Earth. Naturally magnetized pieces of magnetite, called lodestone, will attract small pieces of iron, which is how ancient peoples first discovered the property of magnetism.
"can be magnetized to become a permanent magnet itself."
The mass spectrometer is an instrument which can measure the masses and relative concentrations of atoms and molecules. It makes use of the basic magnetic force on a moving charged particle.
As surface FeII may be oxidized or otherwise unavailable, initial photoactivation may be needed to trigger the Fenton reactivity...
Conclusions
In this work, effective phenol degradation was obtained upon irradiation of magnetite in the presence of H2O2 (heterogeneous photo-Fenton conditions). The initially low reaction rate considerably increased as the reaction progressed, most likely because of: (i) the formation of intermediates such as HQ that are able to reduce FeIII to FeII, which takes part to the Fenton reaction, and (ii) the presence of dissolved Fe due to magnetite dissolution, both in dark and under irradiation.
The need of FeIII reduction, which is also supported by the important role of irradiation, might look surprising when considering that magnetite contains structural FeII. It is thus possible that the latter is not readily available for the Fenton reaction and that some form of initial activation (e.g. partial iron photodissolution) is needed.
On the other hand, phenol degradation was most effectively achieved by the samples having the highest content of structural FeII. The size of the primary particles seems to be less important, because it was not correlated with photo-Fenton activity in the studied samples. However, the size effect could be largely offset by important aggregation phenomena in the aqueous suspensions.
From an applicative point of view, the most interesting features of magnetite as shown in this study are:
Reactivity upon absorption of UVA radiation, which makes cheap sunlight potentially
applicable to carry out the reaction.
Ability of some samples (most notably S2 and S3) to maintain reactivity also under
circumneutral pH conditions, which could save the need to adjust and back-adjust pH.
Limited iron leaching (up to or below the ppm range), which keeps dissolved Fe safely below
the limits for wastewater discharge.
Magnetic behavior, which greatly helps in the separation of magnetite from treated wastewater.