Our current understanding of insect phototransduction is based on a small number of species, but insects occupy many different visual environments. We created the retinal transcriptome of a nocturnal insect, the cockroach, Periplaneta americana to identify proteins involved in the earliest stages of compound eye phototransduction, and test the hypothesis that different visual environments are reflected in different molecular contributions to function.
We assembled five novel mRNAs: two green opsins, one UV opsin, and one each TRP and TRPL ion channel homologs. One green opsin mRNA (pGO1) was 100–1000 times more abundant than the other opsins (pGO2 and pUVO), while pTRPL mRNA was 10 times more abundant than pTRP, estimated by transcriptome analysis or quantitative PCR (qPCR). Electroretinograms were used to record photoreceptor responses. Gene-specific in vivo RNA interference (RNAi) was achieved by injecting long (596–708 bp) double-stranded RNA into head hemolymph, and verified by qPCR.
RNAi of the most abundant green opsin reduced both green opsins by more than 97% without affecting UV opsin, and gave a maximal reduction of 75% in ERG amplitude 7 days after injection that persisted for at least 19 days.
RNAi of pTRP and pTRPL genes each specifically reduced the corresponding mRNA by 90%. Electroretinogram (ERG) reduction by pTRPL RNAi was slower than for opsin, reaching 75% attenuation by 21 days, without recovery at 29 days. pTRP RNAi attenuated ERG much less; only 30% after 21 days. Combined pTRP plus pTRPL RNAi gave only weak evidence of any cooperative interactions. We conclude that silencing retinal genes by in vivo RNAi using long dsRNA is effective, that visible light transduction in Periplaneta is dominated by pGO1, and that pTRPL plays a major role in cockroach phototransduction.
Animal phototransduction proceeds through light absorption by rhodopsins (opsins linked to retinal molecules), which activate ion channels via G-protein coupled second messenger pathways (Fain et al., 2010). Insect and other arthropod compound eyes contain the opsin molecules in microvilli of photoreceptor cells. Rich genetic and molecular tools have made Drosophila compound eyes the best-understood model of insect phototransduction (Hardie and Postma, 2008).
In Drosophila, photon absorption by rhodopsin causes photoisomerization to metarhodopsin, which activates a heterotrimeric Gq-protein, initiating a cascade leading to activation of IP3 and diacylglycerol. Linkages from this cascade to opening of transient receptor potential (dTRP) and TRP-like (dTRPL) ion channels that carry the receptor current are still debated, and both chemical (Chyb et al., 1999; Huang et al., 2010) and mechanical (Hardie and Franze, 2012) intermediate steps have been proposed
In Drosophila, dTRP and dTRPL channels are thought to carry approximately equal parts of light-activated current under physiological conditions (Reuss et al., 1997).
Although major features of Drosophila phototransduction may apply to other insect species, there are probably many variations to accommodate the different visual requirements of this large and diverse group of animals. However, the study of such mechanisms has been hindered by the lack of powerful methods that can be used in Drosophila, including deletion/inactivation mutants and targeted mutations.
Here we used the American cockroach, Periplaneta americana, which has a very different lifestyle to Drosophila, including mainly terrestrial, secluded habitats, reliance on chemical and mechanical information via prominent antennae and cerci, and preference for dark or crepuscular visual environments (Cameron, 1961).
Evidence already exists that the anatomy and physiology of Periplaneta compound eyes are adapted to dim light (Heimonen et al., 2006, 2012), and recent electrophysiological data suggested that these differences include a larger role for TRPL than TRP channels (Immonen et al., 2014).
To explore phototransduction mechanisms we created a transcriptome of Periplaneta retina and assembled mRNA sequences for opsins, TRP and TRPL genes (French, 2012). To investigate the roles of each protein, we used in vivo RNA interference (RNAi) based gene silencing to suppress translation of these genes by injecting double stranded RNA (dsRNA) into the head hemolymph.
Changes in photoreceptor function were measured by an electroretinogram (ERG) assay and the quantity of targeted mRNA measured by qPCR. Our data indicate that Periplaneta retina contains three opsins (pGO1, pGO2, and pUVO), with one of the green opsins, pGO1, dominating vision of visible light. Periplaneta pTRPL mRNA was 10-fold more abundant than pTRP, and RNAi of pTRPL was much more effective in reducing ERG, supporting a more important role for pTRPL than pTRP in Periplaneta phototransduction.
All animal procedures followed protocols approved by the Dalhousie University Committee on Laboratory Animals. Cockroaches, Periplaneta americana, were raised and maintained in the laboratory at a temperature of 22 ± 2°C under a 13 h light/11 h dark cycle.. .
Opsin proteins are fundamental components of animal vision whose structure largely determines the sensitivity of visual pigments to different wavelengths of light. Surprisingly little is known about opsin evolution in beetles, even though they are the most species rich animal group on Earth and exhibit considerable variation in visual system sensitivities. We reveal the patterns of opsin evolution across 62 beetle species and relatives.
Our results show that the major insect opsin class (SW) that typically confers sensitivity to “blue” wavelengths was lost ~300 million years ago, before the origin of modern beetles. We propose that UV and LW opsin gene duplications have restored the potential for trichromacy (three separate channels for colour vision) in beetles up to 12 times and more specifically, duplications within the UV opsin class have likely led to the restoration of “blue” sensitivity up to 10 times.
This finding reveals unexpected plasticity within the insect visual system and highlights its remarkable ability to evolve and adapt to the available light and visual cues present in the environment.
Etymology
tri- + Ancient Greek χρῶμα (khrôma, “color”).
Noun
trichromacy (countable and uncountable, plural trichromacies)
The quality of having three independent channels for conveying color information in the eye.
Trichromacy or trichromatism is the possessing of three independent channels for conveying color information, derived from the three different types of cone cells in the eye. Organisms with trichromacy are called trichromats.
The normal explanation of trichromacy is that the organism's retina contains three types of color receptors (called cone cells in vertebrates) with different absorption spectra. In actuality the number of such receptor types may be greater than three, since different types may be active at different light intensities. In vertebrates with three types of cone cells, at low light intensities the rod cells may contribute to color vision.
Humans and some other mammals have evolved trichromacy based partly on pigments inherited from early vertebrates. In fish and birds, for example, four pigments are used for vision. These extra cone receptor visual pigments detect energy of other wavelengths, sometimes including ultraviolet.
Trichromatic color vision is the ability of humans and some other animals to see different colors, mediated by interactions among three types of color-sensing cone cells.
The trichromatic color theory began in the 18th century, when Thomas Young proposed that color vision was a result of three different photoreceptor cells. From the middle of the 19th century, in his Treatise on Physiological Optics, Hermann von Helmholtz later expanded on Young's ideas using color-matching experiments which showed that people with normal vision needed three wavelengths to create the normal range of colors. Physiological evidence for trichromatic theory was later given by Gunnar Svaetichin (1956).
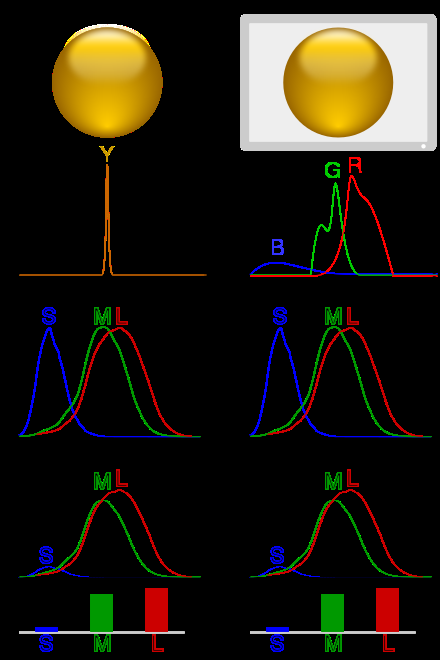
Illustration of colour metamerism:
In column 1, a ball is illuminated by monochromatic light. Multiplying the spectrum by the cones' spectral sensitivity curves gives the response for each cone type.
In column 2, metamerism is used to simulate the scene with blue, green and red LEDs, giving a similar response.
Each of the three types of cones in the retina of the eye contains a different type of photosensitive pigment, which is composed of a transmembrane protein called opsin and a light-sensitive molecule called 11-cis retinal.
Each different pigment is especially sensitive to a certain wavelength of light (that is, the pigment is most likely to produce a cellular response when it is hit by a photon with the specific wavelength to which that pigment is most sensitive). The three types of cones are L, M, and S, which have pigments that respond best to light of long (especially 560 nm), medium (530 nm), and short (420 nm) wavelengths respectively.
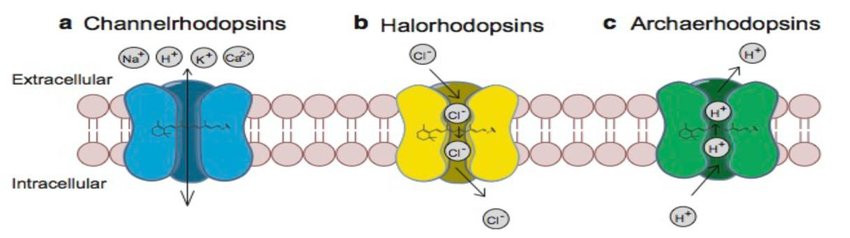
Optogenetics: ↑ A technique that uses a combination of light and genetic engineering to control the activity of a cell. ... Opsins: ↑ Proteins that respond to a specific type of light (for example, ChR2 only responds to blue light). In neuroscience, these proteins are used to control neuron activity.
Since the likelihood of response of a given cone varies not only with the wavelength of the light that hits it but also with its intensity, the brain would not be able to discriminate different colors if it had input from only one type of cone. Thus, interaction between at least two types of cone is necessary to produce the ability to perceive color. With at least two types of cones, the brain can compare the signals from each type and determine both the intensity and color of the light.
Optogenetics is a technique used for the study of neural circuits in the brain. It is a branch of biotechnology that combines genetics and optical techniques to conceive and control a specific neural circuit in a living human brain.
Even though optogenetics is a relatively new neuromodulation tool whose various implications have not yet been scrutinized, it has already been approved for its first clinical trials in humans. 17 Oct 2020
scrutinize (third-person singular simple present scrutinizes, present participle scrutinizing, simple past and past participle scrutinized)
(transitive) To examine something with great care or detail, as to look for hidden or obscure flaws.
to scrutinize the conduct or motives of individuals
(transitive) To audit accounts etc in order to verify them.
To shed light on something...
Viral vectors are tools commonly used by molecular biologists to deliver genetic material into cells. This process can be performed inside a living organism or in cell culture. Viruses have evolved specialized molecular mechanisms to efficiently transport their genomes inside the cells they infect.
"enhanced delivery injection of the viral optogenetic vector"
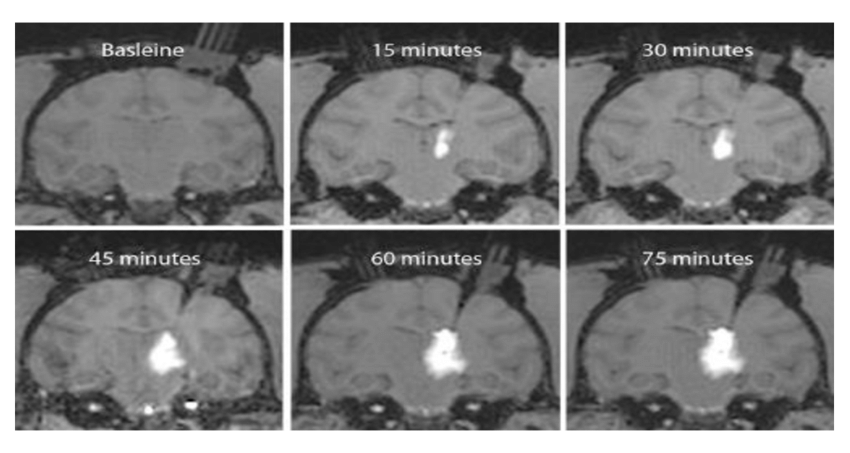
Convection enhanced delivery, or CED, of optogenetic viral vectors in non human primates will enable researchers to manipulate neural activity at a large scale to understand complex neural computations and behaviors. With CED we can achieve high optogenetic expression across large regions of the primate brain with only a few injections in a short amount of time compared to traditional methods.
Magnetic resonance or MR compatible chamber implantation is performed using standard practice in non human primate surgeries, the procedure is described in detail in the text protocol and outlined briefly in this video.
Following animal sedation, positioning, and initial incision as described in the text protocol..
mRNA, or messenger RNA vaccines, borrow a mechanism that all of your cells use to make all the proteins in your body. It helps translate the message in your DNA's genetic code into proteins. 2 days ago
Neuropsin is a protein that in humans is encoded by the OPN5 gene. It is a photoreceptor protein sensitive to ultraviolet (UV) light. ... Neuropsin is bistable at 0 °C and activates a UV-sensitive, heterotrimeric G protein Gi-mediated pathway in mammalian and avian tissues.
Opsins are members of the G protein-coupled receptor superfamily. Human neuropsin is expressed in the eye, brain, testes, and spinal cord. Neuropsin belongs to the seven-exon subfamily of mammalian opsin genes that includes peropsin (RRH) and retinal G protein coupled receptor (RGR). Neuropsin has different isoforms created by alternative splicing