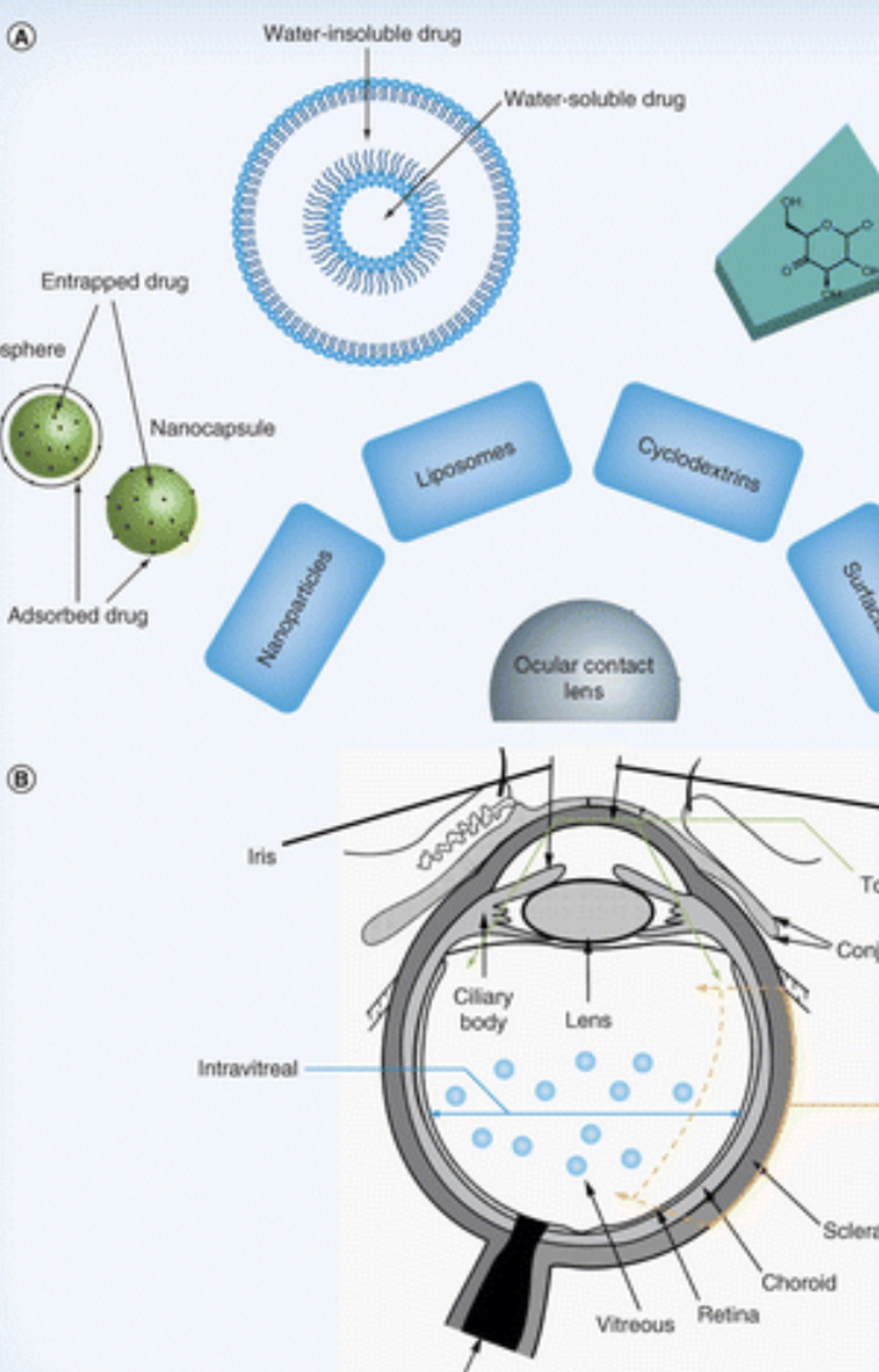
A Schematic drawing illustrating the principle of determination of proximity between ion channels and cell-surface receptors by the patch-clamp technique. Gold nanoparticles of 30 nm diameter bound to the cell-surface receptors, here MHCI, may influence the binding properties of Pi 2 toxin. The modulation of toxin binding properties depend on the size of ligand and nanoparticle, intermolecular distance, and receptor density, as well as the receptor-channel proximity.
Because of the 2D nature of the receptor clusters, the lateral component of toxin transport is more severely hindered than the perpendicular one (length of an Fab or Fc fragment is $6.5 nm; the length of the portion of MHCI or MHCII protruding from the membrane plane is $10 nm). B Illustration of the principle of detection of proximity between two cell-surface receptors (here MHCI and MHCII), one of which (MHCII) when liganded is capable of modulating channel current via some signaling mechanism by retarding ligand (L243 mAb) binding to the ''electrically active'' receptor (MHCII) by a nanoparticle bound to another receptor (MHCI) in close proximity