Ion channels are macromolecular complexes whose functions are exquisitely tuned by interacting proteins. Fluorescence resonance energy transfer (FRET) is a powerful methodology that is adept at quantifying ion channel protein-protein interactions in living cells. For FRET experiments, the interacting partners are tagged with appropriate donor and acceptor fluorescent proteins. If the fluorescently-labeled molecules are in close proximity, then photoexcitation of the donor results in non-radiative energy transfer to the acceptor, and subsequent fluorescence emission of the acceptor
Q follower Arizona #KAG Patriot who loves God and Country #MAGA Trump/JFKJr 2020 #SaveTheChildren Evil Won't Survive in 5D
You didn't include an age restriction on your post.
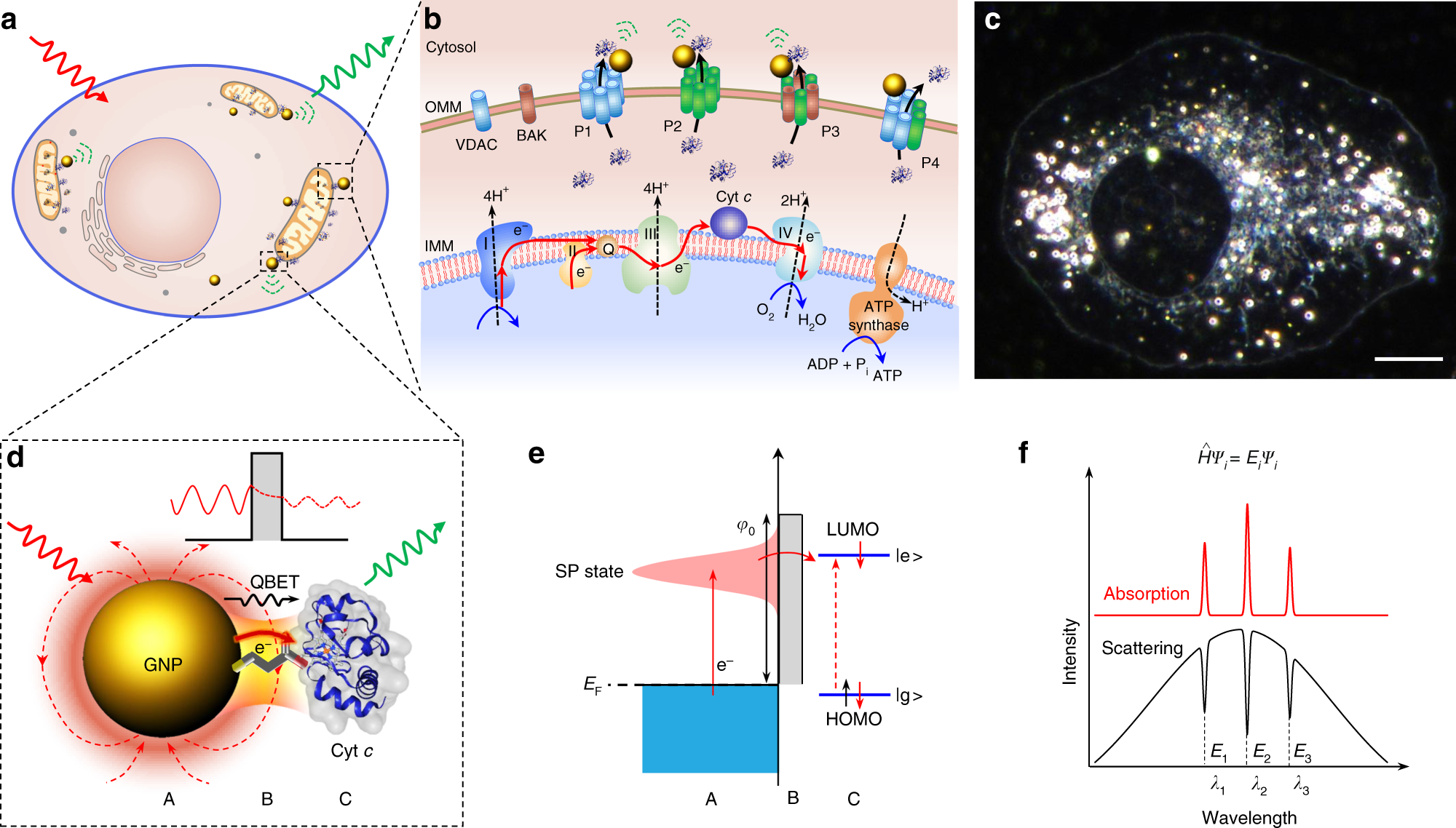
Quantum biological electron transfer (ET) essentially involves in virtually all important biological processes such as photosynthesis, cellular respiration, DNA repair, cellular homeostasis, and cell death. However, there is no real-time imaging method to capture biological electron tunnelling in live cells to date.
Here, we report a quantum biological electron tunnelling (QBET) junction and its application in real-time optical detection of QBET and the dynamics of ET in mitochondrial cytochrome c during cell life and death process. QBET junctions permit to see the behaviours of electron tunnelling through barrier molecules with different barrier widths. Using QBET spectroscopy, we optically capture real-time ET in cytochrome c redox dynamics during cellular apoptosis and necrosis in living cells. The non-invasive real-time QBET spectroscopic imaging of ET in live cell open a new era in life sciences and medicine by providing a way to capture spatiotemporal ET dynamics and to reveal the quantum biological mechanisms.
Q follower Arizona #KAG Patriot who loves God and Country #MAGA Trump/JFKJr 2020 #SaveTheChildren Evil Won't Survive in 5D
I wish Ii understood this; maybe one day I will. From what I understand, we are moving into a new age of enlightenment, new theories and an awakened humanity. Thank you.
I'm starting to think we are on a mostly flat realm with many more "continents" than we were taught. Are those "continents" what science is calling planets?
Your thoughts on that please.
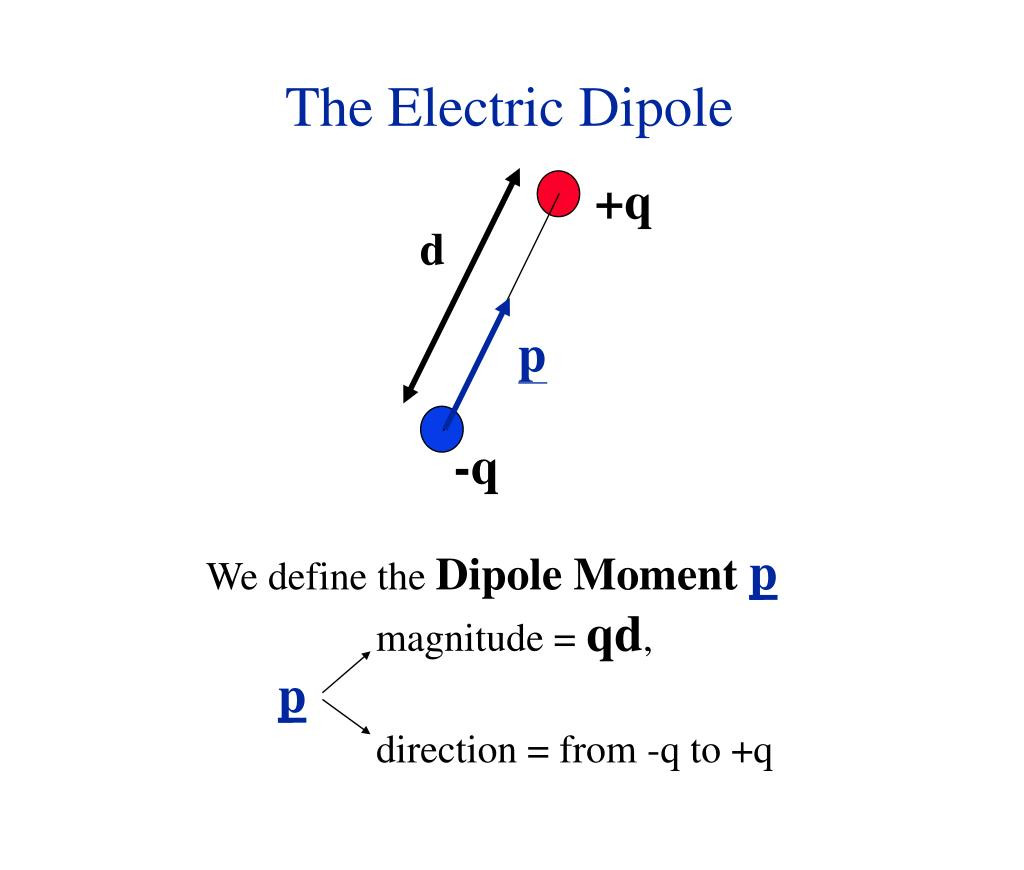
Förster resonance energy transfer (FRET), fluorescence resonance energy transfer, resonance energy transfer (RET) or electronic energy transfer (EET) is a mechanism describing energy transfer between two light-sensitive molecules (chromophores).[1] A donor chromophore, initially in its electronic excited state, may transfer energy to an acceptor chromophore through nonradiative dipole–dipole coupling.